Abstract
Background: In the absence of prophylactic treatment, CNS disease involvement will occur in 30-70% of acute lymphoblastic leukemia (ALL) patients. CNS metastasis is observed in all ALL subtypes, suggesting that lymphoblasts utilize a conserved mechanism to invade the CNS. CNS ALL relapse predicts a poor outcome, but treatment options remain limited due to a minimal understanding of the biologic processes involved.
PI3K regulates in many cell activities, including proliferation, migration, and apoptosis. The δ isoform of PI3K is preferentially expressed in immune cells. The PI3Kδ inhibitor, idelalisib, is approved for use in combination with rituximab for treatment of chronic lymphocytic leukemia. Previously, the PI3Kd tool compound, GS-649443 (443), which has more favorable murine pharmacokinetic properties, was evaluated in in vivo models of acute lymphoblastic leukemia (Yao H, et al. ASH meeting, 2016). Here we report on the mechanisms of ALL CNS invasion and the potential for PI3Kd inhibition to block CNS disease.
Results: Our prior study of 443 in Nalm-6 (N6) B-ALL mice demonstrated that PI3Kd inhibition prevents ALL CNS disease progression. All control mice showed symptoms of CNS involvement including hind limb paralysis, but only 17% of 443-treated mice developed paralysis at their clinical endpoint. There was no difference in ALL burden or apoptotic rate in bone marrow (BM) or spleen of treated vs. control mice at matched time points. In contrast, cerebrospinal fluid (CSF) blast counts were significantly decreased in 443-treated mice.
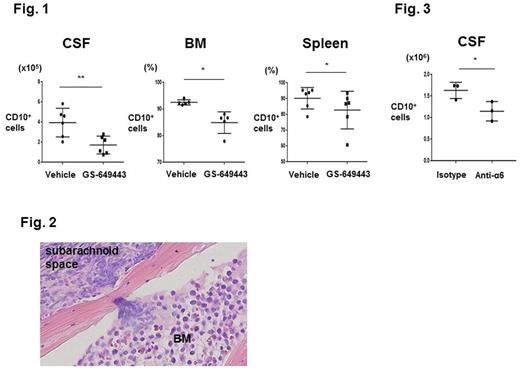
To confirm our results were not isolated to the N6 model, we tested the efficacy of PI3Kδ inhibition in mice engrafted with primary human ALL (hALL). Treated mice demonstrated a modest decrease in peripheral disease burden. Similar to the results in N6 mice, 443 markedly decreased CNS disease burden in the hALL model (Fig. 1).
Examination of 443 pharmacokinetics as well as mitotic and apoptotic indices of CSF blasts isolated from 443 vs. vehicle-treated mice indicated that PI3Kd inhibition did not directly impact growth of ALL in the CNS. We therefore investigated whether 443 prevented migration of blasts from the periphery into the CNS. We found that 443 decreased Transwell® (TW) migration of N6 and hALL cells toward SDF-1 or human CSF. We next interrogated the signaling pathways downstream of PI3K that are implicated in cell motility and found that 443 suppressed the phosphorylation of myosin light chain 2 in ALL cells. To identify candidate regulatory molecules upstream of this pathway, we performed a microarray analysis of N6 cells isolated from GS-443 vs. vehicle-treated mice. This showed that multiple genes within focal adhesion and contractility pathways were downregulated in vivo in 443 treated blasts, including integrin α6.
α6, a subunit of cell surface laminin receptors, is expressed in the majority of ALL cases. Laminin deposition is found in the extracellular matrix (ECM) of CNS microvessels and meninges, where it coordinates α6-dependent neuronal progenitor cell pathfinding. Using confocal microscopy, we determined that N6 and hALL cells in circulation are unable to breach the blood brain barrier. Rather, we identified that ALL cells migrate into the CNS along vessels that pass directly between vertebral or calvarial BM and the subarachnoid space, and that the ECM of these bridging vessels is enriched in laminin (Fig. 2). In in vitro invasion assays, we confirmed that ALL migration to CSF is enhanced by laminin in an α6-dependent manner. Lastly, the in vivo effects of α6 were investigated in the N6 model. Mice were treated with a6 neutralizing antibodies beginning on post-engraftment day 1 through the development of clinical symptoms requiring sacrifice. Anti-α6-treated mice showed cachexia at their clinical endpoint but none had severe CNS symptoms, whereas 100% of isotype control-treated mice showed hind limb paralysis. No difference in ALL burden or apoptotic rate was observed in the BM or spleen of anti-α6 vs. isotype control groups. In contrast, there was a significant decrease in the number of blasts harvested from the CSF of anti-α6-treated mice (Fig. 3).
Conclusion: Our results identify a novel ALL metastasis mechanism. Expression of integrin α6, common in ALL, allows cells to co-opt neural migratory pathways to invade the CNS. The potential for PI3Kδ inhibition to block development of CNS ALL warrants further investigation in a clinical study.
Yao: Gilead Sciences, Inc.: Research Funding. Warner: Gilead Sciences, Inc.: Research Funding. Price: Gilead Sciences, Inc.: Research Funding. Olivere: Gilead Sciences, Inc.: Research Funding. Cantelli: Gilead Sciences, Inc.: Research Funding. Ridge: Gilead Sciences, Inc.: Research Funding. Ngo: Gilead Sciences, Inc.: Research Funding. Ravichandran: Gilead Sciences, Inc.: Research Funding. Tannheimer: Gilead Sciences, Inc.: Employment. Sipkins: Gilead Sciences, Inc.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal